Question 1:
Write names.
a. Alloy of sodium with mercury.
b. Molecular formula of the common ore of aluminium.
c. The oxide that forms salt and water by reacting with both acid and base.
d. The device used for grinding an ore.
e. The nonmetal having electrical conductivity.
f. The reagent that dissolves noble metals.
ANSWER:
a. Sodium amalgam, commonly denoted as Na(Hg), it is an alloy of mercury and sodium.
b. Bauxite(Al2O3.2H2O) is the common ore of aluminium.
c. Metal oxides which react with both acids as well as bases to produce salts and water are also known as amphoteric oxides. For example: Al2O3 is an amphoteric oxide. An amphoteric compound is a molecule or ion that can react both an acid as well as a base to produce salt and water.
d. The device used for grinding an ore is grinding mill.
e. Graphite an allotrope of carbon is a good conductor of electricity.
f. Aqua regia is 1:3 mixture of concentrated nitric and hydrochloric acids. It dissolves noble metals such as gold, palladium, and platinum.
Question 2:
Make pairs of substances and their properties
Substance
Property
a. Potassium bromide
1. Combustible
b. Gold
2. Soluble in water
c. Sulphur
3. No chemical reaction
d. Neon
4. High ductility.
Substance
|
Property
|
a. Potassium bromide
|
1. Combustible
|
b. Gold
|
2. Soluble in water
|
c. Sulphur
|
3. No chemical reaction
|
d. Neon
|
4. High ductility.
|
ANSWER:
Substance
Property
a. Potassium bromide
1. Soluble in water
b. Gold
2. High ductility
c. Sulphur
3. Combustible
d. Neon
4. No chemical reaction
Substance
|
Property
|
a. Potassium bromide
|
1. Soluble in water
|
b. Gold
|
2. High ductility
|
c. Sulphur
|
3. Combustible
|
d. Neon
|
4. No chemical reaction
|
Question 3:
Identify the pairs of metals and their ores from the following.
Group A
Group B
a. Bauxite
i. Mercury
b. Cassiterite
ii. Aluminium
c. Cinnabar
iii. Tin
Group A
|
Group B
|
a. Bauxite
|
i. Mercury
|
b. Cassiterite
|
ii. Aluminium
|
c. Cinnabar
|
iii. Tin
|
ANSWER:
Group A
Group B
a. Bauxite
i. Aluminium
b. Cassiterite
ii. Tin
c. Cinnabar
iii. Mercury
Group A
|
Group B
|
a. Bauxite
|
i. Aluminium
|
b. Cassiterite
|
ii. Tin
|
c. Cinnabar
|
iii. Mercury
|
Question 4:
Explain the terms.
a. Metallurgy
b. Ores
c. Minerals
d. Gangue
ANSWER:
a. Metallurgy: The various processes involved in the extraction of metals from its ores and refining is called metallurgy.
The major steps involved for the extraction of a metal from its ore are:
(i) Concentration of ores (or enrichment of ore)
(ii) Conversion of concentrated ore into metal
(iii) Refining (purification) of impure metal
b. Ores: Those minerals from which metals can be extracted conveniently and profitably.
c. Minerals:
"A mineral is an element or chemical compound that is normally crystalline and that has been formed as a result of geological processes" (Nickel, E. H., 1995).
"Minerals are naturally-occurring inorganic substances with a definite and predictable chemical composition and physical properties." (O' Donoghue,1990).
"A mineral is a naturally occurring homogeneous solid, inorganically formed, with a definite chemical composition and an ordered atomic arrangement" (Mason, et al,1968).
"These... minerals ...can be distinguished from one another by individual characteristics that arise directly from the kinds of atoms they contain and the arrangements these atoms make inside them" (Sinkankas, 1966).
"A mineral is a body produced by the processes of inorganic nature, having usually a definite chemical composition and, if formed under favorable conditions, a certain characteristic atomic structure which is expressed in its crystalline form and other physical properties" (Dana & Ford,1932).
"Every distinct chemical compound occurring in inorganic nature, having a definite molecular structure or system of crystallization and well-defined physical properties, constitutes a mineral species" (Brush & Penfield
d. Gangue is the unwanted impurities like rock material, dust, soil, sand, earthy particles, limestone, mica etc. present in an ore.
Question 5:
Write scientific reasons.
a. Lemon or tamarind is used for cleaning copper vessels turned greenish.
b. Generally the ionic compounds have high melting points.
c. Sodium is always kept in kerosene.
d. Pine oil is used in froth flotation.
e. Anodes need to be replaced from time to time during the electrolysis of alumina.
ANSWER:
a. Copper vessels turned greenish due to the formation of copper carbonate layer. The citric acid present in the lemon or tamarind neutralises the basic copper carbonate and dissolves the layer. That is why, tarnished copper vessels are cleaned with lemon or tamarind juice to give the surface of the copper vessel its characteristic lustre.
b. In an ionic compound there is strong electrostatic force of attraction between oppositely charged ions. To overcome these forces a considerable amount of energy is needed. Therefore, ionic compounds have high melting points.
c. Sodium is a very reactive metal. It is kept in kerosene to prevent it from coming in contact with oxygen and moisture. If this happens, it will react with the moisture present in air and form sodium hydroxide. This is a strongly exothermic reaction and lot of heat is generated.
d. Pine oil is added in the froth flotation method to create froth or bubble so that metal can be purify easily because pine oil prevents the ore from gangue for further mixing. Pine oil also acts as the best substance for forming froth for the minerals. It also increases the non wettability of mineral particles.
e. In the electrolysis of alumina, graphite rod is used as anode.
During the electrolytic reduction of alumina, aluminium is produced at the cathode and oxygen gas is evolved at the anode.
This gas reacts with the graphite rods (anode) and forms carbon dioxide.
Thus, the graphite rods are continuously eroded. Hence, the graphite rods i.e. anodes be replaced from time to time during the electrolysis of fused alumina.
Question 6:
When a copper coin is dipped in silver nitrate solution, a glitter appears on the coin after some time. Why does this happen? Write the chemical equation.
ANSWER:
Copper is more reactive than silver. Hence, displacement reaction occurs. When copper coin is dipped in silver nitrate solution, it forms copper nitrate and silver metal. A shining white deposit of silver metal is formed on copper coin. The grey solid crystal(glitters) of Ag metal appears on the copper coin and solution turns blue in colour.
Balanced equation:
2AgNO3(aq) + Cu(s) → Cu(NO3)2(aq) + 2Ag(s)
Question 7:
The electronic configuration of metal ‘A’ is 2,8,1 and that of metal ‘B’ is 2,8,2. Which of the two metals is more reactive? Write their reaction with dilute hydrochloric acid.
ANSWER:
On moving from left to right in a period of periodic table, the chemical reactivity of elements first decreases from sodium to silicon and then increases from phosphorus to chlorine.
The electronic configuration of metal ‘A’ is 2,8,1. This is electronic configuration of sodium metal.
The electronic configuration of metal ‘B’ is 2,8,2. This is electronic configuration of magnesium metal.
In the first element of third period, sodium, there is 1 valence electron which it can lose easily to react with other substances, so it is very reactive metal. The second element magnesium has 2 valence electrons. It is not easy for an atom to lose 2 electrons, so magnesium is less reactive than sodium.
Reaction with dil hydrochloric acid:
Question 8:
Draw a neat labelled diagram.
a. Magnetic separation method.
ANSWER:
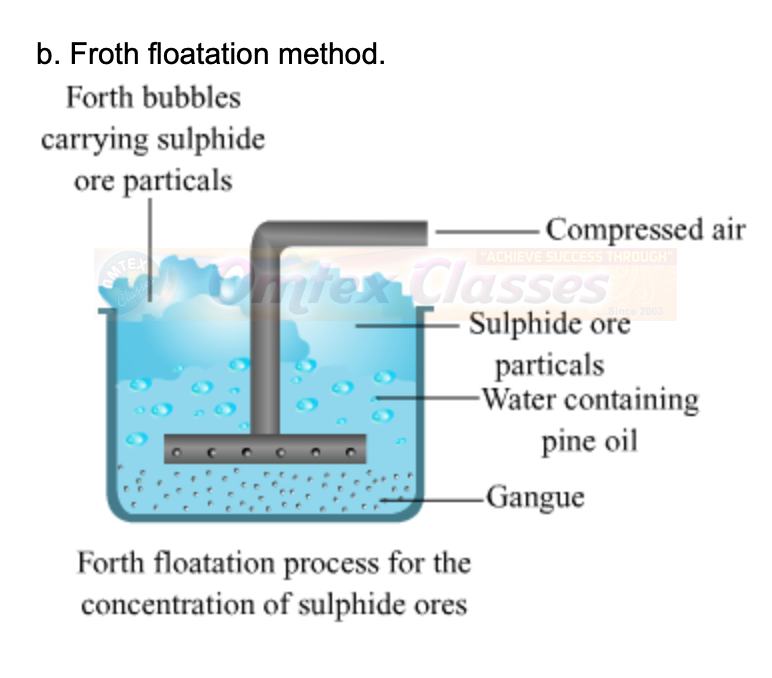
 b. Froth floatation method.
b. Froth floatation method.
ANSWER:
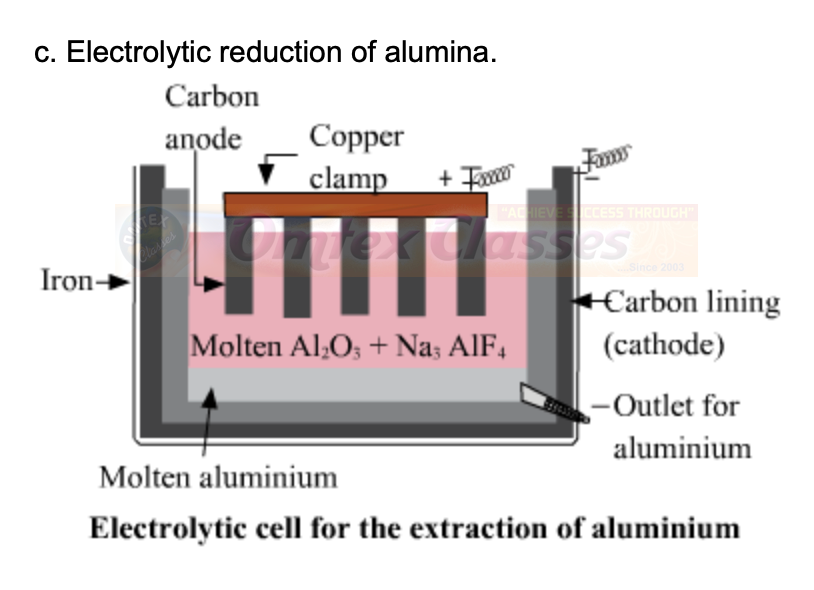
 c. Electrolytic reduction of alumina.
c. Electrolytic reduction of alumina.
ANSWER:
 d. Hydraulic separation method.
d. Hydraulic separation method.
ANSWER:
Question 9:
Write the chemical equation for the following events.
a. Aluminium came in contact with air.
b. Iron filings are dropped in aqueous solution of copper sulphate.
c. A reaction was brought about between ferric oxide and aluminium.
d. Electrolysis of alumina is done.
e. Zinc oxide is dissolved in dilute hydrochloric acid.
ANSWER:
a. Aluminium came in contact with air.
Aluminum is a very reactive metal. The outer surface of the metal is actually covered by a very thin layer of the oxide which keeps the metal protected from the air. But when the oxide layer is damaged, aluminum comes in contact with the air. It is easily attacked by air. Then aluminium starts reacting with the oxygen. It will burn as bright white flame to change into aluminum(III) oxide.
Chemical Equation:
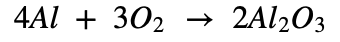 b. Iron filings are dropped in aqueous solution of copper sulphate.
Iron is more reactive than copper. It can displace Copper (Cu) from its salt Copper sulphate (CuSO4) and its colour changes from blue to green.
Chemical Equation:
b. Iron filings are dropped in aqueous solution of copper sulphate.
Iron is more reactive than copper. It can displace Copper (Cu) from its salt Copper sulphate (CuSO4) and its colour changes from blue to green.
Chemical Equation:
 c. A reaction was brought about between ferric oxide and aluminium.
Aluminium is more reactive than iron. Aluminium metal replaces iron from ferric oxide to form aluminium oxide and iron
Chemical Equation:
c. A reaction was brought about between ferric oxide and aluminium.
Aluminium is more reactive than iron. Aluminium metal replaces iron from ferric oxide to form aluminium oxide and iron
Chemical Equation:
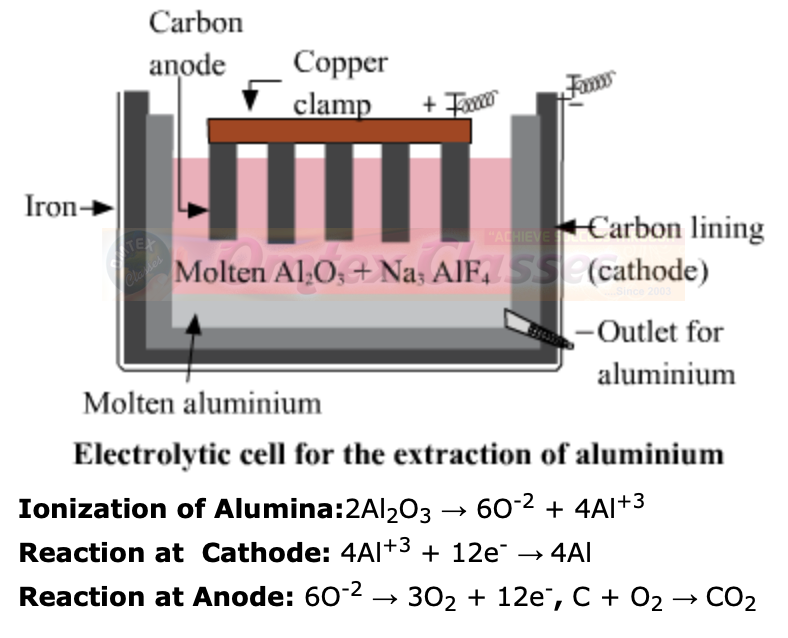
 d. Electrolysis of alumina is done.
The electrolysis of alumina is carried out in a steel tank lined inside with graphite. The graphite lining serves as cathode. Anode is also made up of graphite rods hanging in the molten mass. The electrolyte consists of alumina dissolved in fused Cryolite(Na3AlF6) and Fluorspar(CaF2). Cryolite lowers the melting point of alumina and fluorspar increases the fluidity of the mass so that the liberated aluminum metal may sink at the bottom of the cell. When electric current is passed through this mixture, the aluminum is collected at the cathode in molten state and sinks at the bottom.
d. Electrolysis of alumina is done.
The electrolysis of alumina is carried out in a steel tank lined inside with graphite. The graphite lining serves as cathode. Anode is also made up of graphite rods hanging in the molten mass. The electrolyte consists of alumina dissolved in fused Cryolite(Na3AlF6) and Fluorspar(CaF2). Cryolite lowers the melting point of alumina and fluorspar increases the fluidity of the mass so that the liberated aluminum metal may sink at the bottom of the cell. When electric current is passed through this mixture, the aluminum is collected at the cathode in molten state and sinks at the bottom.
 e. Zinc oxide is dissolved in dilute hydrochloric acid.
Zinc Oxide is an inorganic compound with the formula ZnO. It is insoluble in water. When Zinc oxide reacts with hydrochloric acid it forms zinc chloride and water. It also leads in the formation of small bubbles of hydrogen. This is a double displacement reaction.
Chemical Equation:
e. Zinc oxide is dissolved in dilute hydrochloric acid.
Zinc Oxide is an inorganic compound with the formula ZnO. It is insoluble in water. When Zinc oxide reacts with hydrochloric acid it forms zinc chloride and water. It also leads in the formation of small bubbles of hydrogen. This is a double displacement reaction.
Chemical Equation:
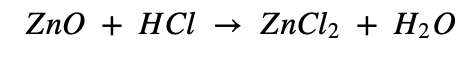
Question 10:
Complete the following statement using every given options.
During the extraction of aluminium..............
a. Ingredients and gangue in bauxite.
b. Use of leaching during the concentration of ore.
c. Chemical reaction of transformation of bauxite into alumina by Hall’s process.
d. Heating the aluminium ore with concentrated caustic soda.
ANSWER:
a. Bauxite is the main ore of aluminium. Silica (SiO2), ferric oxide (Fe2O3) and titanium oxide (TiO2) are the impurities present in bauxite.
Ingredient in bauxite are molten cryolite (Na3AIF6), fluorspar (CaF2).
b. The separation of impurities(silica (SiO2), ferric oxide (Fe2O3) and titanium oxide (TiO2)) in bauxite ore is done by leaching process using either Bayer’s method or Hall’s method.
c. In the Hall’s process the bauxite is in powdered form and then leached by heating with aqueous sodium carbonate in the digester to form water soluble sodium aluminate. Then the insoluble impurities are filtered out. The filtrate is warmed and neutralised by passing carbon dioxide gas through it. This results in the precipitation of aluminium hydroxide.
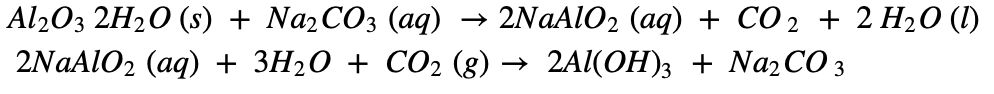 The precipitate of Al(OH)3 obtained in both, Bayer’s and Hall’s processes is filtered, washed, dried and then calcined by heating at 10000C to obtain alumina.
The precipitate of Al(OH)3 obtained in both, Bayer’s and Hall’s processes is filtered, washed, dried and then calcined by heating at 10000C to obtain alumina.
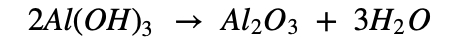 d. When aluminium ore is heated with caustic soda (NaOH) solution under high pressure for 2-8 hours at 1400-1500C, water soluble sodium aluminate is formed.
d. When aluminium ore is heated with caustic soda (NaOH) solution under high pressure for 2-8 hours at 1400-1500C, water soluble sodium aluminate is formed.

Question 11:
Divide the metals Cu, Zn, Ca, Mg, Fe, Na, Li into three groups, namely reactive metals, moderately reactive metals and less reactive metals.
ANSWER:
Highly reactive metals
moderately reactive metals
less reactive metals.
Ca
Fe
Au
Mg
Zn
Ag
Na
Cu
Pt
Li
Highly reactive metals
|
moderately reactive metals
|
less reactive metals.
|
Ca
|
Fe
|
Au
|
Mg
|
Zn
|
Ag
|
Na
|
Cu
|
Pt
|
Li
|